1. Mechanosensitive remodeling of adherens junctions
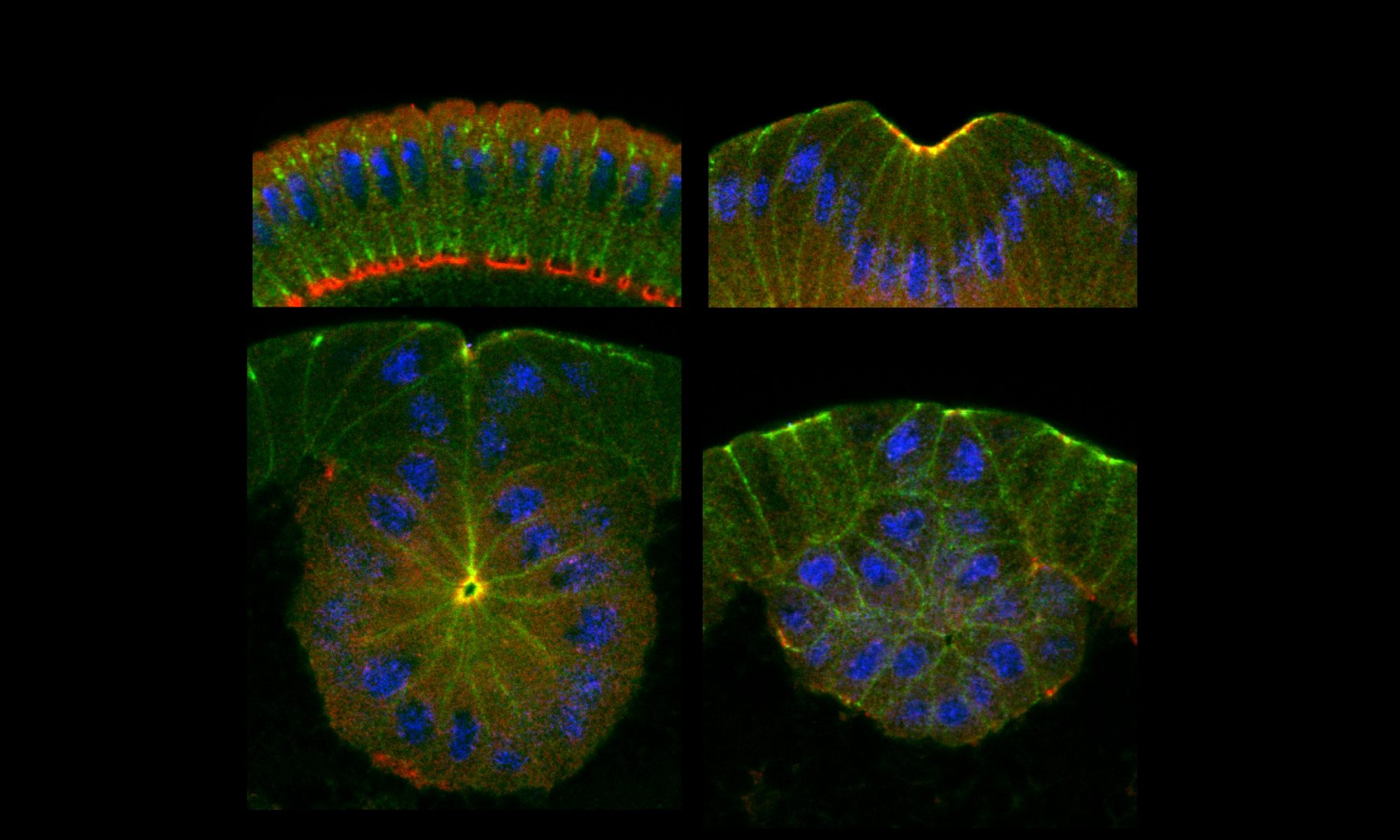
We have established fly mesoderm primordium as a great system to study mechanosensitive response of adherens junctions. At this stage of development, adherens junctions are the only junctions in the cell therefore are at the front line to experience physical tension. In addition, due to the expression of transcriptional factor Snail in the mesoderm, polarity proteins, which in most systems provide the major mechanism in maintaining junctions, are downregulated. The loss of this polarity-based mechanism render adherens junctions more sensitive to changes in mechanical tension and thus the dependence of junctions on tensions is more easily detected. Combined with the genetic tools to manipulate endogenous myosin levels and the advantage of live imaging, we seek to elucidate the molecular mechanisms underlying the mechanosensitive remodeling of adherens junctions.

Mesodermal cells in all animals need to be internalized inside to become internal tissues such as bone, muscle, blood, and so on. In fly embryos, they invaginate through epithelial folding. This is driven by the actomyosin contractile network on the apical surface of those cells. Laser cutting experiments indicate high tensions across the mesoderm epithelia. To keep tissue integrity under such levels of tension, strong adherens junctions are essential in holding cells together and compromised adherens junctions leads to tissue tear. Our previous study showed that, in response to the activation of myosin contraction upon gastrulation, adherens junctions change from elongated clusters distributed at the subapical position into highly concentrated spots at the very apical edge of the cell. Tracing of individual junction clusters reveal that junction clusters persist and migrate towards the apical direction while grow in densities and sizes. These changes start simultaneously with myosin activation and are temporarily correlated with individual myosin pulses. Myosin loss of function analysis indicates that these junctional changes depends on myosin activity. It is not clear how myosin contraction reorganize ECadherin molecules inside the junctions. Is it through membrane protein turnover or physical sequestering? Is it through reorganizing actin cytoskeleton or recruitment of tension-sensitive junction binding partners? Is the repositioning through molecular treadmilling or physical displacement? These are the questions we are interested in.
2. Mechanosensitive regulation of EMT progression

Epithelial-mesenchymal transition (EMT) is a process where cells loose cell-cell junctions and apical-basal polarities, leave the epithelial sheet, acquire migratory ability to become founder cells of a new colony, often of different cell types. This process drives the emergence of generation of three germ layers during gastrulation and is employed by the cancer cells to disseminate. Despite the simple concept, more and more recent studies have pointed to a model where EMT is not a binary switch and there are intermediate stops along the epithelial to mesenchymal axis, which appears to be more relavant to physiological plasticity and cancer metastasis.
In fly embryos, mesoderm EMT is driven by the conserved transcriptional factor Snail, a master EMT driver. We observe that Snail is expressed very early on during development and drive the declining of polarity protein Par3/Bazooka and subsequently adherens junctions prior to gastrulation. However cells cannot be disassociated before the mesoderm is internalized, as they are internalized through myosin-driven epithelial folding and loss of cell-cell adhesions will leads to tissue tear during this tension-intensive process. We found that by maintaining and remodeling of adherens junctions, mechanical tensions triumph the actions of EMT driver Snail in regulating the level of adherens junctions. This mechanism reverses the Snail-driven declining of adherens junctions prior to gastrulation and is released only after mesoderm cells are completely internalized and myosin is no longer activated. We hypothesize that mechanosensitive regulation of adherens junctions may provide a mechanism to stabilize the cells at an intermediate state during EMT and maintains the selected features of epithelial cells while allow the development of other mesenchymal characters.
3. Post-translational regulations of adherens junctions and polarity protein Par3/Bazooka during EMT
Downregulation of junctional components is one of the major function of Snail during EMT however we find that the conventional transcriptional repression of ECadherin gene is not sufficient for junction loss in fly early embryos. In fact ECadherin and other junctional components are maternally provided in the early embryos and so are not susceptible to transcriptional regulation. In addition, we observe that Snail expression is only sufficient to remove adherens junction structure but has no effect on other pools of junctional components, such as the membrane diffusing pool and the basal junctions at the cellularization front. We identified Par3/Bazooka as the target of Snail as Snail is necessary and sufficient to drive loss of Par3/Bazooka from junctional sites in mesoderm while loss of Par3/Bazooka phenocopies Snail gain-of-functions. Because Par3/Bazooka is also maternally provided we are in the process of identifying Snail transcriptional target genes factors that is responsible for the mesoderm-specific downregulation of Par3/Bazooka.